PROSTATEx | SPIE-AAPM-NCI PROSTATEx Challenges
DOI: 10.7937/K9TCIA.2017.MURS5CL | Data Citation Required | 7.1k Views | 98 Citations | Image Collection
| Location | Species | Subjects | Data Types | Cancer Types | Size | Status | Updated | |
|---|---|---|---|---|---|---|---|---|
| Prostate | Human | 346 | MR, Other, Classification | Prostate Cancer, Non-Cancer | Image Analyses | Public, Complete | 2022/07/05 |
Summary
The ProstateX dataset (both training and testing cases) have been included in the PI-CAI Public Training and Development dataset. As such, ProstateX as a benchmark has been deprecated and is superseded by the PI-CAI challenge. PI-CAI is an all-new grand challenge, with over 10,000 carefully-curated prostate MRI exams to validate modern AI algorithms and estimate radiologists' performance at clinically significant prostate cancer detection and diagnosis. Key aspects of the study design have been established in conjunction with an international, multi-disciplinary scientific advisory board (16 experts in prostate AI, radiology and urology) - to unify and standardize present-day guidelines, and to ensure meaningful validation of prostate-AI towards clinical translation. Please refer to https://pi-cai.grand-challenge.org for more information. The PROSTATEx Challenge ("SPIE-AAPM-NCI Prostate MR Classification Challenge”) focused on quantitative image analysis methods for the diagnostic classification of clinically significant prostate cancers and was held in conjunction with the 2017 SPIE Medical Imaging Symposium. PROSTATEx ran from November 21, 2016 to January 15, 2017, though a "live" version has also been established at https://prostatex.grand-challenge.org which serves as an ongoing way for researchers to benchmark their performance for this task. The PROSTATEx-2 Challenge ("SPIE-AAPM-NCI Prostate MR Gleason Grade Group Challenge" ) ran from May 15, 2017 to June 23, 2017 and was focused on the development of quantitative multi-parametric MRI biomarkers for the determination of Gleason Grade Group in prostate cancer. It was held in conjunction with the 2017 AAPM Annual Meeting (see http://www.aapm.org/GrandChallenge/PROSTATEx-2). Supplemental data and instructions specific to both challenges are in the Detailed Description section below. This collection is a retrospective set of prostate MR studies. All studies included T2-weighted (T2W), proton density-weighted (PD-W), dynamic contrast enhanced (DCE), and diffusion-weighted (DW) imaging. The images were acquired on two different types of Siemens 3T MR scanners, the MAGNETOM Trio and Skyra. T2-weighted images were acquired using a turbo spin echo sequence and had a resolution of around 0.5 mm in plane and a slice thickness of 3.6 mm. The DCE time series was acquired using a 3-D turbo flash gradient echo sequence with a resolution of around 1.5 mm in-plane, a slice thickness of 4 mm and a temporal resolution of 3.5 s. The proton density weighted image was acquired prior to the DCE time series using the same sequence with different echo and repetition times and a different flip angle. Finally, the DWI series were acquired with a single-shot echo planar imaging sequence with a resolution of 2 mm in-plane and 3.6 mm slice thickness and with diffusion-encoding gradients in three directions. Three b-values were acquired (50, 400, and 800), and subsequently, the ADC map was calculated by the scanner software. All images were acquired without an endorectal coil.PROSTATEx has been superseded by PI-CAI:
Accessing the PROSTATEx Challenge Data Sets
Image Acquisition Details
Data Access
Note: The following download links will download the entire collection. See the Detailed Description section of this page for information about subsets specific to the PROSTATEx challenges.
Version 2: Updated 2022/07/05
Added Test Reference Standard document
| Title | Data Type | Format | Access Points | Subjects | License | Metadata | |||
|---|---|---|---|---|---|---|---|---|---|
| Download images | MR | DICOM | Download requires NBIA Data Retriever |
346 | 349 | 18,321 | 309,251 | CC BY 3.0 | View |
| Ktrans images, Lesion Info and Thumbnails | Other | DOCX, DICOM, MHD, ZIP, BMP, and CSV | Download requires IBM-Aspera-Connect plugin |
344 | 6,690 | CC BY 3.0 | — | ||
| Test set reference standard | Classification | CSV | 140 | CC BY 3.0 | — |
Additional Resources for this Dataset
The NCI Cancer Research Data Commons (CRDC) provides access to additional data and a cloud-based data science infrastructure that connects data sets with analytics tools to allow users to share, integrate, analyze, and visualize cancer research data.
- Imaging Data Commons (IDC) (Imaging Data)
- IDC Zenodo community dataset: Image segmentations produced by BAMF under the AIMI Annotations initiative
Citations & Data Usage Policy
Data Citation Required: Users must abide by the TCIA Data Usage Policy and Restrictions. Attribution must include the following citation, including the Digital Object Identifier:
Data Citation |
|
|
Litjens, G., Debats, O., Barentsz, J., Karssemeijer, N., & Huisman, H. (2017). SPIE-AAPM PROSTATEx Challenge Data (Version 2) [dataset]. The Cancer Imaging Archive. https://doi.org/10.7937/K9TCIA.2017.MURS5CL |
Detailed Description
PROSTATEx Challenge (November 21, 2016 to February 16, 2017)
SPIE, along with the support of the American Association of Physicists in Medicine (AAPM) and the National Cancer Institute (NCI), conducted a “Grand Challenge” on quantitative image analysis methods for the diagnostic classification of clinically significant prostate lesions. For more details, go to https://prostatex.grand-challenge.org/ .
Training and test cohorts along with supplemental Ktrans images and lesion information can be obtained separately using the following options for download:
| Data Type | Training Cohort
(204 subjects) |
Test Cohort
(140 subjects) |
|---|---|---|
| Download images (DICOM, 126 kB) (DICOM, 121 kB) | ||
| Download Ktrans images (.mhd 161 kB) (.mhd 119 kB) | ||
| Download lesion information (.docx, .csv, .zip, 281 kB) (.docx, .csv, .zip, 236 kB) | ||
| Download lesion reference thumbnails (.bmp, 37.6 MB .zip) (.bmp, 23.3 MB .zip) |
The images come in two encodings. The acquired MR is provided in DICOM encoding. Additionally Ktrans images are provided. They come in mhd format. Ktrans is a key pharmacokinetic parameter computed from the available Dynamic contrast enhanced T1-weighted series. Each patient has one study with several DICOM images and one Ktrans image. The Ktrans image is encoded in two files ProstateX-[ProxID]-Ktrans.[mhd/zraw], where ProxID is the ProstateX patient identifier. The DICOM images comprise several Series each comprising several Instances. The DICOM files are documented in the ProstateX-Images.csv file. The columns in that file encode the following:
- ProxID – ProstateX patient identifier.
- Name – Series Description
- Studydate – Study Date
- fid – Finding ID
- Pos – Scanner Coordinate position of the finding
- WorldMatrix – Matrix describing image orientation and scaling
- ijk – image col,row,slice coordinate of finding
- ImageUID – Image Identifier
- TopLevel
- 0 – Series forms one image
- 1 – A set of Series forms a 4D image (e.g. Dynamic MR)
- NA – Series form one image, but is part of a Level 1 4D image
- SpacingBetweenSlices – Scalar Spacing between slices
- VoxelSpacing – Vector with x,y,z spacing scalars
- Dim – Vector with 4D dimensions of the image
- DCMSerDescr – The original DICOM Series Description
- DCMSerUID – The DICOM Series UID
- DCMSerNum – The DICOM Series Number
- InstanceUIDList – DICOM Instances that make up this series
- ImageUIDList – TopLevel-NA Images the make up this Toplevel 1 image
For example, to get the ADC image of Patient ProstateX-0123 do the following. After you imported the DICOM files into your environment, go to patient ProstateX-0123 and find the series with ADC in it. In this case it is ‘ep2d_diff_tra_DYNDIST_ADC’. It has SeriesNumber 8. The DICOM images in that series form the ADC image for this challenge. Image slice j at coordinate i,j contains a finding fid. See Findings for more details.
Findings
The findings are documented in the ProstateX-Findings.csv table. Documentation for the columns in that table is as follows:
- ProxID – ProstateX patient identifier
- fid – Finding ID
- pos – Scanner Coordinate position of the finding
- ClinSig – Identifier available in training set that identifies whether this is a clinically significant finding. Either the biopsy GleasonScore was 7 or higher. Findings with a PIRADS score 2 were not biopsied and are not considered clinically significant. In our center the occurrence of clinically significant cancer in PIRADS 2 lesions is less than 5%.
Note, ProxID is the PROSTATEx case ID, and fid is the finding (i.e., lesion) ID in both the ProstateX-Findings.csv file and the ProstateX-Images.csv file. The Findings spreadsheet has one row per lesion (if a case has only one lesion, then the only fid for that case will be “1” , alternately if a case has two lesions, then there will be an fid of “1” and an fid of “2” for that case). The Images spreadsheet has a row for every image that contains the lesion – that is why there are multiple rows with the same (ProxID, fid) combination.
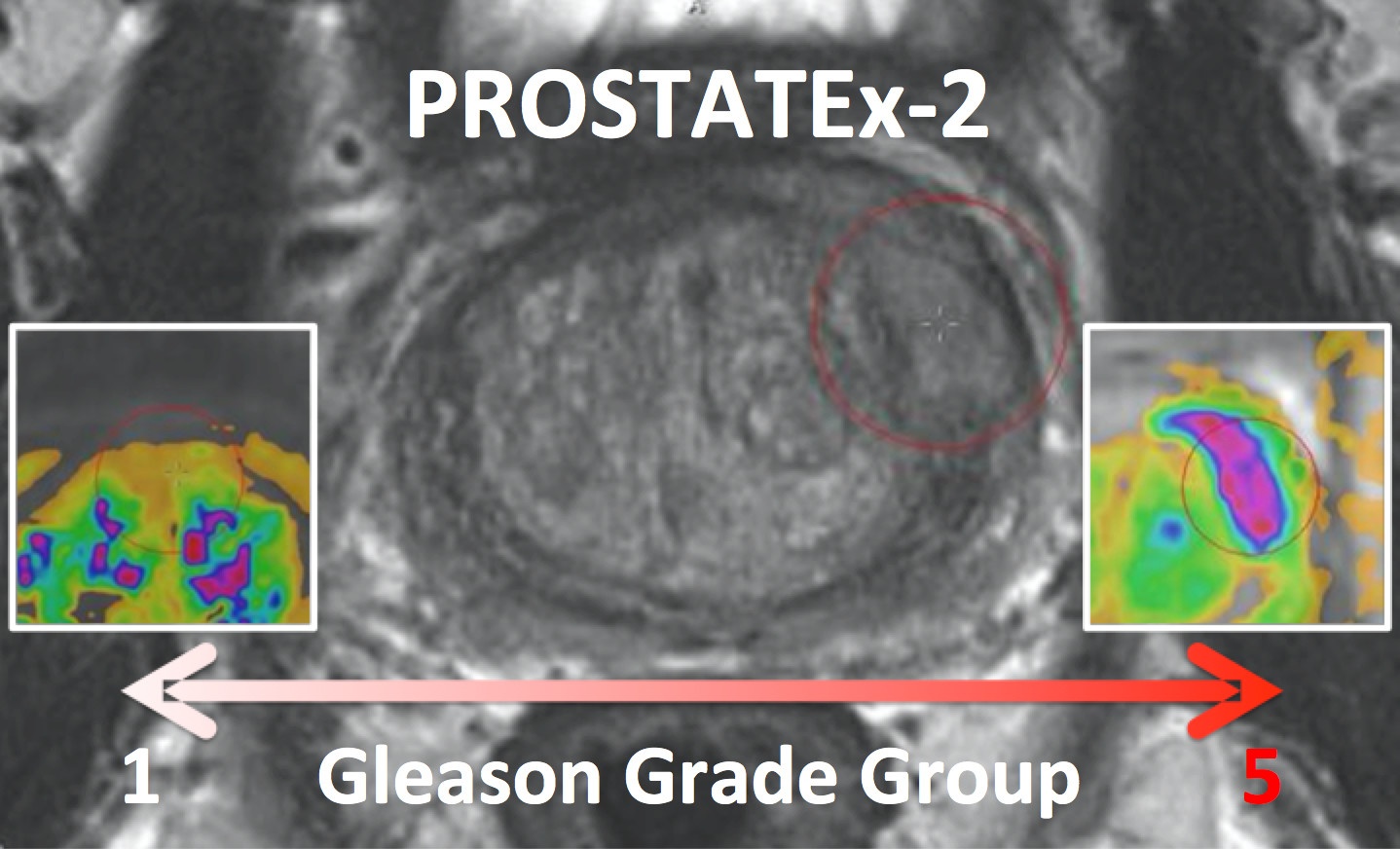
PROSTATEx-2 — SPIE-AAPM-NCI Prostate MR Gleason Grade Group Challenge (May 15, 2017 to August 3, 2017)
The American Association of Physicists in Medicine (AAPM), along with the SPIE (the international society for optics and photonics) and the National Cancer Institute (NCI), conducted a part 2 “Grand Challenge” on the development of quantitative multi-parametric magnetic resonance imaging (MRI) biomarkers for the determination of Gleason Grade Group in prostate cancer. For more details about PROSTATEx-2 please go to http://www.aapm.org/GrandChallenge/PROSTATEx-2/default.asp.
Training and test cohorts along with supplemental Ktrans images and lesion information can be obtained separately using the following options for download:
| Data Type | Training Cohort
(112 subjects) |
Test Cohort
(70 subjects) |
|---|---|---|
| Download images (DICOM) | ||
| Download Ktrans images (.mhd, .zraw) | ||
| Download lesion information (.docx, .csv, .zip) | ||
| Download lesion reference thumbnails (.bmp, .zip) |
The training set will consist of 112 findings. This dataset will be representative of the technical properties (scanner type, acquisition parameters, file format) and the nature of the prostate lesions of the test set. An associated Excel file will include case name, the coordinates of the centroid of all lesions, and the lesion ‘truth’ label (Gleason Grade Group). Reference thumbnail images of the lesions will also be provided.
The test set will consist of 70 findings. The locations of the lesions will be specified in the accompanying Excel file that will follow the same format as for the training set with the omission of the ‘truth’ labels. Reference thumbnail images of the lesions will also be provided.
Acknowledgements
The prostate MR imaging was performed at the Radboud University Medical Centre (Radboudumc) in the Prostate MR Reference Center under supervision of prof. Dr. Barentsz. The Radboudumc is located in Nijmegen, The Netherlands. The dataset was collected and curated for research in computer aided diagnosis of prostate MR under supervision of Dr. Huisman, Radboudumc.
Related Publications
Publications by the Dataset Authors
The authors recommended the following as the best source of additional information about this dataset:
Publication Citation |
|
|
Litjens, G., Debats, O., Barentsz, J., Karssemeijer, N., & Huisman, H. (2014). Computer-Aided Detection of Prostate Cancer in MRI. In IEEE Transactions on Medical Imaging (Vol. 33, Issue 5, pp. 1083–1092). Institute of Electrical and Electronics Engineers (IEEE). https://doi.org/10.1109/tmi.2014.2303821 |
No other publications were recommended by dataset authors.
Research Community Publications
TCIA maintains a list of publications that leveraged this dataset. If you have a manuscript you’d like to add please contact TCIA’s Helpdesk.
