ISPY1 | Multi-center breast DCE-MRI data and segmentations from patients in the I-SPY 1/ACRIN 6657 trials
DOI: 10.7937/K9/TCIA.2016.HdHpgJLK | Data Citation Required | Image Collection
| Location | Species | Subjects | Data Types | Cancer Types | Size | Status | Updated | |
|---|---|---|---|---|---|---|---|---|
| Breast | Human | 222 | SEG, MR | Breast Cancer | Clinical, Image Analyses | Public, Complete | 2016/09/28 |
Summary
ACRIN 6657 was designed as a prospective study to test MRI for ability to predict response to treatment and risk-of-recurrence in patients with stage 2 or 3 breast cancer receiving neoadjuvant chemotherapy (NACT). ACRIN 6657 was conducted as a companion study to CALGB 150007, a correlative science study evaluating tissue-based biomarkers in the setting of neoadjuvant treatment of breast cancer. Collectively, CALGB 150007 and ACRIN 6657 formed the basis of the multicenter Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and moLecular Analysis (I-SPY TRIAL) breast cancer trial, a study of imaging and tissue-based biomarkers for predicting pathologic complete response (pCR) and recurrence-free survival (RFS). Additional information about the trial is available in the Study Protocol and Case Report Forms. Participant Eligibility and Enrollment: Criteria for inclusion were patients enrolling on CALGB 150007 with T3 tumors measuring at least 3 cm in diameter by clinical exam or imaging and receiving neoadjuvant chemotherapy with an anthracycline-cyclophosphamide regimen alone or followed by a taxane. Pregnant patients and those with ferromagnetic prostheses were excluded from the study. The study was open to enrollment from May 2002 to March 2006. 237 patients were enrolled, of which 230 met eligibility criteria.
Data Access
The ISPY team has provided additional options for download. The significance and download links for these subsets are explained under Detailed Description.
Version 2: Updated 2016/09/28
Data publicly released and new “level-specific” download options provided.
| Title | Data Type | Format | Access Points | Subjects | License | |||
|---|---|---|---|---|---|---|---|---|
| Images and Segmentations | SEG, MR | DICOM | Download requires NBIA Data Retriever |
222 | 847 | 9,032 | 387,680 | CC BY 3.0 |
| DICOM Metadata Digest | CSV | CC BY 3.0 | ||||||
| Clinical and Outcome Data | CSV | CC BY 3.0 |
Additional Resources for this Dataset
The NCI Cancer Research Data Commons (CRDC) provides access to additional data and a cloud-based data science infrastructure that connects data sets with analytics tools to allow users to share, integrate, analyze, and visualize cancer research data.
- Imaging Data Commons (IDC) (Imaging Data)
Citations & Data Usage Policy
Data Citation Required: Users must abide by the TCIA Data Usage Policy and Restrictions. Attribution must include the following citation, including the Digital Object Identifier:
Data Citation |
|
|
David Newitt, Nola Hylton, on behalf of the I-SPY 1 Network and ACRIN 6657 Trial Team. (2016). Multi-center breast DCE-MRI data and segmentations from patients in the I-SPY 1/ACRIN 6657 trials. The Cancer Imaging Archive. https://doi.org/10.7937/K9/TCIA.2016.HdHpgJLK |
Detailed Description
Requirements for MR imaging (As specified in the ACRIN 6657 protocol)
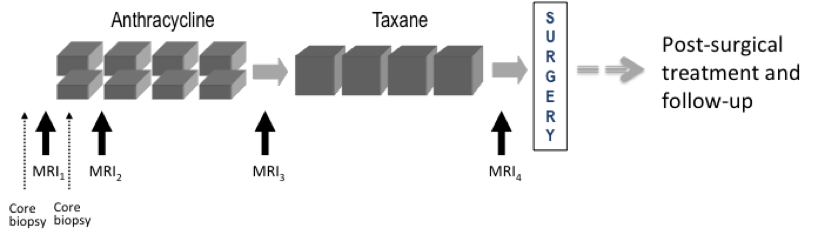
Imaging time points: MRI exams were performed within four weeks prior to starting anthracycline-cyclophosphamide chemotherapy (T1, MRI1), at least 2 weeks after the first cycle of AC and prior to the second cycle of AC (T2, MRI2), between anthracycline-cyclophosphamide treatment and taxane therapy if taxane was administered (T3, MRI3), and after the final chemotherapy treatment and prior to surgery (T4, MRI4). The study schema is shown in Figure 1
Figure 1. CALGB 150007 and ACRIN 6657 study schema.
Imaging protocol: MR imaging was performed on a 1.5 Tesla field strength scanner using a dedicated breast radiofrequency coil. The image acquisition protocol included a localization scan and T2-weighted sequence followed by a contrast-enhanced T1-weighted series. All imaging was performed unilaterally over the symptomatic breast and in the sagittal orientation. The contrast-enhanced series consisted of a high resolution (≤1mm in-plane spatial resolution) three-dimensional, fat-suppressed, T1-weighted gradient echo sequence with TR≤20 ms, TE = 4.5 ms, flip angle ≤ 45º, 16-18 cm field-of-view, minimum matrix 256×192, 64 slices, slice thickness ≤ 2.5 mm. Scan time length for the T1-weighted sequence was required to be between 4.5 and 5 minutes. The sequence was acquired once before contrast injection and repeated at least twice following injection.
Tumor diameter measurement and volumetric analysis: Tumor longest diameter (LD) was measured by the site radiologist as the greatest extent of disease on baseline MR images, including intervening areas of non-enhancing tissue. The same measurement direction was used on all subsequent MRI exams. The primary predictor variable, functional tumor volume (FTV) was measured from contrast-enhanced images using the signal enhancement ratio (SER) method. Volumetric analysis, including Quality Control assessment, was performed centrally at the Breast Imaging Research Program (BIRP) laboratory at University of California at San Francisco (UCSF).
Detailed information about the DICOM data is available in the DICOM Dictionary.
Further information on these studies can be found at:
- ACRIN 6657 Protocol https://www.acr.org/Research/Clinical-Research/ACRIN-Legacy-Trials
- CALGB 150007 https://clinicaltrials.gov/study/NCT01042379
Imaging Data Transfer History
The processing of the MR image data for ACRIN 6657 consisted of the following steps between image acquisition and the creation of this shared data set on TCIA:
- MRI studies were sent from the study centers to the ACRIN Core Lab either via media (DVD) or the TRIAD program
- Image data were de-identified and centrally archived at the ACRIN Core Lab
- Archived data was sent to the Breast Imaging Research Program (BIRP) at the University of California, San Francisco (UCSF) for volumetric analysis.
- De-identified image data, derived analysis maps and segmentations, and ancillary data files were transferred from UCSF to TCIA for data sharing.
While every effort was made to preserve the integrity of both the original image data and image meta-data (DICOM attributes, public and private), multiple file transfers and strict adherence to HIPPA guidelines for patient confidentiality may have resulted in loss of some data. If any questions arise, or patient PHI is found in any data on this collection, please contact the UCSF Breast Imaging Research Program (BIRP). For scientific or other inquiries about this dataset, please contact TCIA’s Helpdesk.
Curated Data Sets
In addition to the complete set of ACRIN 6657 imaging studies (“Level 0” data), the following curated data sets based on UCSF QC assessment, protocol compliance and data completeness are provided:
- Level 1: MRI Longest Diameter (LD)
- Level 2a: SER Volume Dataset for ongoing volumetric analyses (updated 9/17/16)
- Level 2b: SER Volume Dataset for pCR Analysis (Hylton, et al; Radiology 2012)
- Level 3: SER Volume Dataset for RFS Analysis (Hylton, et al; Radiology, 2016)
The image data sets are accompanied by Excel files with selected patient clinical and outcome data.
Data subset Descriptions
| Data set | subjects | All Series | DCE + Derived Only | DCE Only | Clinical and outcome data |
|---|---|---|---|---|---|
| Level 0: Complete image data set | 222 | NA | NA | ||
| Level 1: Studies with MRI LD measurements | 219 | NA | NA | ||
| Level 2a: Studies with SER Volume measurements | 207 | ||||
| Level 3: Studies used in primary aim analysis | 162 |
Level 0: Complete I-SPY 1 / ACRIN 6657 MRI Dataset
This data set is comprised of all HIPAA compliant, DICOM compliant MRI series.
Level 0 image data set consists of 847 on-study MRI studies on 222 subjects in the UCSF image database.
One patient in the image data collection (I-SPY ID 1079) does not appear in the Feb. 2, 2011 I-SPY FINAL LOCKED clinical data dump. So no clinical or outcome data is available for this subject.
Level 1: MRI exams for which longest diameter was measured
This data set is comprised of all studies with MRI measured longest diameter (LD) values reported.
839 MRI studies have LD reported in the I-SPY 1 clinical database, of which 5 studies are not present in either the UCSF or ACRIN image data collections (see Table 1).
Level 1 image data set consists of 834 MRI studies on 219 subjects in the UCSF image database
| Table 1. Studies that have LD measurement but are missing from
the UCSF and ACRIN TRIAD image data collections: |
||
|---|---|---|
| ID 1071, T1 ID 1138, T1 |
ID 1101, T3 ID 1040, T4 |
ID 1187, T4 |
Level 2a: Good SER Volume Dataset – updated 9/3/14, 9/17/16
This data set is comprised of the patient studies which, following quality reviews in 2014 and 2016, were judged to have sufficiently good image quality and protocol compliance for volumetric DCE SER analysis. Rejection criteria included: incomplete volumetric DCE acquisitions, lack of a 2nd post-contrast acquisition, variability in fat suppression across the image, observed patient motion during the DCE acquisition, significant DCE protocol deviations such as changing scan parameters or image position during DCE acquisition.
Level 2a image data set consists of 706 MR studies on 207 subjects in the UCSF image database. These include 7 studies not included in Level 1 (no MRI LD recorded) as listed in Table 2.
| Table 2. Studies in Level 2a (good volumetric analysis) that do NOT have LD measures: | ||
|---|---|---|
| ID 1059, T4 ID 1079, T2 * ID 1104, T4 |
ID 1192, T2 ID 1212, T4 |
ID 1215, T1 ID 1238, T2 |
| * Patient 1079 does not appear in the Feb. 2, 2011 I-SPY FINAL LOCKED clinical data set. So no clinical or outcome data is available. | ||
Level 2b: SER Volume Dataset Reported in Hylton et al. (Radiology, 2012)
This data set is comprised of the patient studies analyzed for pCR outcome and reported in the 2012 Radiology paper on ACRIN 6657 pCR results *. This data set is not provided as a shared list, as it is not recommended for use in further analysis. It is described here because it is the data set from which the Level 3 (primary aim analysis) set was derived. Inclusion and exclusion was determined by quality and protocol reviews available at that time. In addition to the exclusion criteria listed for Level 2a, studies done with imaging in the axial plane, in violation of the sagittal orientation specified in the trial imaging protocol, were excluded due to processing limitations of the analysis software. Similarly, bi-lateral sagittal acquisitions (alternating left and right volumetric acquisitions) were excluded.
Level 2b image data set consists of 707 MRI studies on 207 subjects in the UCSF image database.
Tables 3 and 4 show the specific inclusion/exclusion differences between Levels 2a and 2b:
| Table 3. 16 studies accepted for SER analysis since 2008 (in Level 2a but not in 2b) | ||
|---|---|---|
| ID 1005, T3 ID 1043, T2 ID 1046, T4 ID 1057, T3 ID 1074, T3 ID 1084, T1 |
ID 1110, T4 ID 1139, T4 ID 1159, T4 ID 1176, T2 ID 1201, T2 |
ID 1203, T4 ID 1206, T4 ID 1225, T3 ID 1219, T3 ID 1228, T4 |
| Table 4. 17 studies rejected since 2008 (in Level 2b but not in 2a) | |
| Study ID and TP | Reason for rejection for volumetric SER analysis (level 2a) |
|---|---|
| 1007 T4 * | No fatSat; Different protocol from T1 |
| 1035 T4 * | Only 1 post scan then acq. parameters changed |
| 1045 T1 | Alternating laterality acquisitions, 2 minute time gap |
| 1047 T1 * | Image position changed during DCE |
| 1053 T2 * | Alternating laterality acquisitions, bad pre- acquisition |
| 1053 T4 * | Alternating laterality acquisitions |
| 1055 T1 * | Alternating laterality acquisitions, 4 minute time gap |
| 1086 T1 * | Alternating laterality acquisitions, time gap, different protocol from T4 |
| 1091 T1 * | Changing acq. parameters during DCE |
| 1095 T2 * | Only 1 post scan then acq. parameters changed |
| 1173 T3 * | Off protocol timing |
| 1206 T1 | Bad DCE timing, 20 minute delay |
| 1206 T2 | Bad DCE timing, 1’29” acquisition time |
| 1224 T3 * | Scan position changed during DCE |
| 1230 T3 * | Scan position changed during DCE |
| #128 T1, T2 | 2 studies for ineligible patient: ACRIN ID 128 (no I-SPY ID) |
| * Subjects that were included in the primary aim analysis (Level 3) | |
Level 3: Subset of Level 2b used in primary aim analysis, reported in Hylton et al. (Radiology, 2016) *
This data set is comprised of the patient studies analyzed for RFS outcome and reported in the 2015 Radiology paper on ACRIN 6657 survival results (Hylton et al, Radiology *). Table 5 shows the 45 patients excluded from the level 2a cohort for this analysis. Please see the publication for specific information on exclusions of patients from this group.
Level 3 image data set consists of 586 MRI studies on 162 subjects in the UCSF image database. This is also the study cohort used as the Test Phase data in the QIN BMMR Challenge.
| Table 5. 45 subjects excluded from Level 2b set | |||||
|---|---|---|---|---|---|
| ID 1027
ID 1040 ID 1045 ID 1046 ID 1048 ID 1054 ID 1063 ID 1067 |
ID 1079
ID 1084 ID 1103 ID 1110 ID 1120 ID 1137 ID 1139 ID 1152 |
ID 1157
ID 1159 ID 1160 ID 1167 ID 1171 ID 1176 ID 1177 ID 1180 |
ID 1182
ID 1185 ID 1187 ID 1189 ID 1192 ID 1194 ID 1203 ID 1206 |
ID 1210
ID 1212 ID 1214 ID 1215 ID 1219 ID 1221 ID 1222 ID 1228 |
ID 1234
ID 1235 ID 1237 ID 1238 ineligible: Case #: 128 |
Acknowledgements
This shared data set was provided by David Newitt, PhD and Nola Hylton, PhD from the Breast Imaging Research Program at UCSF, in collaboration with ACRIN, CALGB, the I-SPY TRIAL, and TCIA. Many thanks are due to The ACRIN 6657 trial team, The I-SPY 1 TRIAL team, and all the patients participating in these studies.
Funding sources include NIH grants to UCSF (R01 CA132870 and U01 CA151235), ACRIN (UO1 CA079778 and UO1 CA080098), and CALGB (UO1 CA31964 and UO1 CA33601).
Related Publications
Publications by the Dataset Authors
Publication Citation |
|
|
Hylton, N. M., Gatsonis, C. A., Rosen, M. A., Lehman, C. D., Newitt, D. C., Partridge, S. C., Bernreuter, W. K., Pisano, E. D., Morris, E. A., Weatherall, P. T., Polin, S. M., Newstead, G. M., Marques, H. S., Esserman, L. J., & Schnall, M. D. (2016). Neoadjuvant Chemotherapy for Breast Cancer: Functional Tumor Volume by MR Imaging Predicts Recurrence-free Survival—Results from the ACRIN 6657/CALGB 150007 I-SPY 1 TRIAL. In Radiology (Vol. 279, Issue 1, pp. 44–55). Radiological Society of North America (RSNA). https://doi.org/10.1148/radiol.2015150013 |
Research Community Publications
The Collection authors suggest the below will give context to this dataset:
- Hylton, N. M., Blume, J. D., Bernreuter, W. K., Pisano, E. D., Rosen, M. A., Morris, E. A., Weatherall, P. T., Lehman, C. D., Newstead, G. M., Polin, S., Marques, H. S., Esserman, L. J., & Schnall, M. D. (2012). Locally Advanced Breast Cancer: MR Imaging for Prediction of Response to Neoadjuvant Chemotherapy—Results from ACRIN 6657/I-SPY TRIAL Radiology. https://doi.org/10.1148/radiol.12110748 PMC3359517
- Hylton, N. M., Gatsonis, C. A., Rosen, M. A., Lehman, C. D., Newitt, D. C., Partridge, S. C., Bernreuter, W. K., Pisano, E. D., Morris, E. A., Weatherall, P. T., Polin, S. M., Newstead, G. M., Marques, H. S., Esserman, L. J., & Schnall, M. D. (2016). Neoadjuvant Chemotherapy for Breast Cancer: Functional Tumor Volume by MR Imaging Predicts Recurrence-free Survival—Results from the ACRIN 6657/CALGB 150007 I-SPY 1 TRIAL. https://doi.org/10.1148/radiol.2015150013 PMC4819899
TCIA maintains a list of publications which leverage our data.
- Al-Tashi, Q., Saad, M. B., Sheshadri, A., Wu, C. C., Chang, J. Y., Al-Lazikani, B., . . . Wu, J. (2023). SwarmDeepSurv: swarm intelligence advances deep survival network for prognostic radiomics signatures in four solid cancers. Patterns. doi: 10.1016/j.patter.2023.100777.
-
Cattell, R. F., Kang, J. J., Ren, T., Huang, P. B., Muttreja, A., Dacosta, S., . . . Duong, T. Q. (2019). MRI volume changes of axillary lymph nodes as predictor of pathological complete responses to neoadjuvant chemotherapy in breast cancer. Clinical Breast Cancer. doi: 10.1016/j.clbc.2019.06.006.
-
Chitalia, R., Pati, S., Bhalerao, M., Thakur, S. P., Jahani, N., Belenky, V., . . . Bakas, S. (2022). Expert tumor annotations and radiomics for locally advanced breast cancer in DCE-MRI for ACRIN 6657/I-SPY1. Sci Data, 9(1), 440. doi: 10.1038/s41597-022-01555-4.
-
Comes, M. C., Fanizzi, A., Bove, S., Didonna, V., Diotaiuti, S., La Forgia, D., . . . Massafra, R. (2021). Early prediction of neoadjuvant chemotherapy response by exploiting a transfer learning approach on breast DCE-MRIs. Sci Rep, 11(1), 14123. doi: 10.1038/s41598-021-93592-z.
-
Drukker, K., Edwards, A., Papaioannou, J., Giger, M., Hahn, H. K., & Mazurowski, M. A. (2020). Long short-term memory networks predict breast cancer recurrence in analysis of consecutive MRIs acquired during the course of neoadjuvant chemotherapy. Paper presented at the SPIE Medical Imaging, Houston TX USA. doi: 10.1117/12.2549044.
-
Du, R., & Vardhanabhuti, V. (2020, 06-08 July 2020). 3D-RADNet: Extracting labels from DICOM metadata for training general medical domain deep 3D convolution neural networks. Paper presented at the Third Conference on Medical Imaging with Deep Learning (MIDL 2020), Montréal, QC, Canada. Retrieved from https://proceedings.mlr.press/v121/du20a.html.
-
Duanmu, H., Huang, P. B., Brahmavar, S., Lin, S., Ren, T., Kong, J., . . . Duong, T. Q. (2020). Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer Using Deep Learning with Integrative Imaging, Molecular and Demographic Data. In Medical Image Computing and Computer Assisted Intervention – MICCAI 2020 (Vol. 12262, pp. 242-252). Lima, Peru: Springer. doi: 10.1007/978-3-030-59713-9_24.
-
Fan, M., Xia, P., Liu, B., Zhang, L., Wang, Y., Gao, X., & Li, L. (2019). Tumour heterogeneity revealed by unsupervised decomposition of dynamic contrast-enhanced magnetic resonance imaging is associated with underlying gene expression patterns and poor survival in breast cancer patients. Breast Cancer Res, 21(1), 112. doi: 10.1186/s13058-019-1199-8.
-
Gierlinger, M., Brandner, D., & Zagar, B. G. (2021, March 17th – 18th, 2021). Vessel extraction from breast MR. Paper presented at the OCM 2021-Optical Characterization of Materials, KARLSRUHE | GERMANY. doi: 10.5445/KSP/1000128686.
-
Jahani, N., Cohen, E., Hsieh, M.-K., Weinstein, S. P., Pantalone, L., Hylton, N., . . . Kontos, D. (2019). Prediction of Treatment Response to Neoadjuvant Chemotherapy for Breast Cancer via Early Changes in Tumor Heterogeneity Captured by DCE-MRI Registration. Sci Rep, 9(1), 12114. doi: 10.1038/s41598-019-48465-x.
-
Kang, J., Li, H., Cattell, R., Talanki, V., Cohen, J. A., Bernstein, C. S., & Duong, T. (2020). The contribution of axillary lymph node volume to recurrence-free survival status in breast cancer patients with sub-stratification by molecular subtypes and pathological complete response. Breast Cancer Research. doi: 10.21203/rs.3.rs-57680/v1.
-
Li, R., & Chen, X. (2022). An efficient interactive multi-label segmentation tool for 2D and 3D medical images using fully connected conditional random field. Comput Methods Programs Biomed, 213, 106534. doi: 10.1016/j.cmpb.2021.106534.
-
Massafra, R., Comes, M. C., Bove, S., Didonna, V., Gatta, G., Giotta, F., . . . Paradiso, A. V. (2022). Robustness Evaluation of a Deep Learning Model on Sagittal and Axial Breast DCE-MRIs to Predict Pathological Complete Response to Neoadjuvant Chemotherapy. Journal of Personalized Medicine, 12(6). doi: 10.3390/jpm12060953.
-
Moyya, P. D., Asaithambi, M., & Ramaniharan, A. K. (2022). Progesterone Receptor Status Analysis in Breast Cancer Patients using DCE- MR Images and Gabor Derived Anisotropy Index. Paper presented at the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy. doi: 10.1109/MeMeA54994.2022.9856476.
-
Nave, O. (2020). Adding features from the mathematical model of breast cancer to predict the tumour size. International Journal of Computer Mathematics: Computer Systems Theory, 5(3), 159-174. doi: 10.1080/23799927.2020.1792552.
-
Pati, S., Thakur, S. P., Hamamcı, İ. E., Baid, U., Baheti, B., Bhalerao, M., . . . Bakas, S. (2023). GaNDLF: the generally nuanced deep learning framework for scalable end-to-end clinical workflows. Communications Engineering, 2(1). doi: 10.1038/s44172-023-00066-3.
Altmetrics
If you have a manuscript you’d like to add please contact TCIA’s Helpdesk.