Advanced-MRI-Breast-Lesions | Standard and Delayed Contrast-Enhanced MRI of Malignant and Benign Breast Lesions with Histological and Clinical Supporting Data
DOI: 10.7937/C7X1-YN57 | Data Citation Required | 4.4k Views | 1 Citations | Image Collection
| Location | Species | Subjects | Data Types | Cancer Types | Size | Status | Updated | |
|---|---|---|---|---|---|---|---|---|
| Breast | Human | 632 | MR, SEG, Histopathology, Molecular Test, Classification, Demographic | Breast Cancer | Clinical | Public, Complete | 2024/04/15 |
Summary
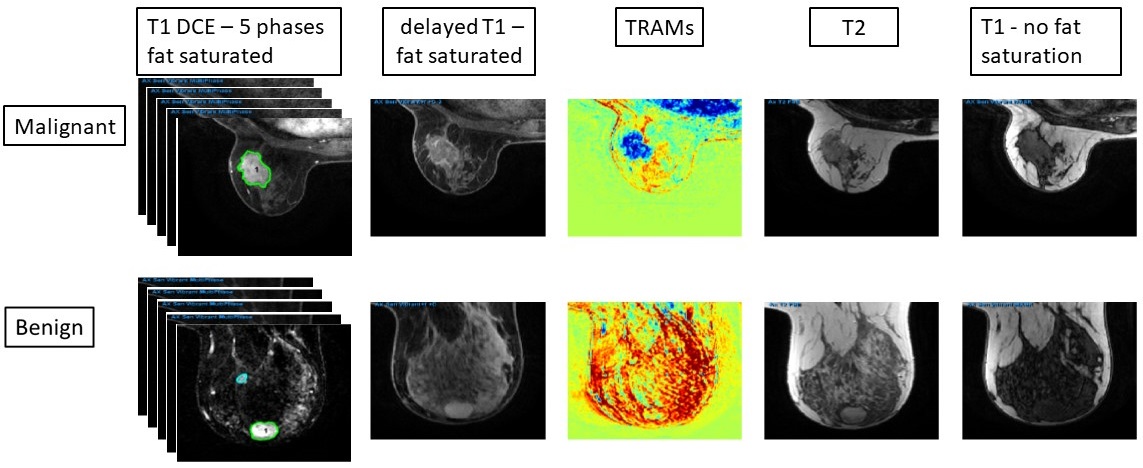
The application of deep-learning methods to breast MRI has a potential to improve diagnosis as well as predict pathological features of the tumors and their response to therapy, and is currently widely investigated. Standard contrast-enhanced MRI of the breast provides high sensitivity but variable specificity in detecting breast cancer, and may lead to excessive benign biopsies. Large, well annotated datasets are needed to improve the current results in this field. This dataset is a single-institutional, retrospective collection of 632 breast-MRI imaging sessions acquired on a 1.5T MR system between 2018-2021. Supporting data, collected for 200 patients from clinical notes, radiology reports and pathology reports, is included: patient age, lesion location, malignant/benign outcome, pathology and grade (if applicable), receptor status and KI67 (if applicable). All enhancing/suspicious positions that were mentioned in the radiological reports were first delineated manually by a certified radiologist with 23 years of experience. Then, these regions were thresholded automatically, keeping the pixels in which the intensity of the first/second subtracted images (subtraction of the pre-contrast image from the first/second post-contrast image) were above the threshold of 75. The main uniqueness of this data is that it contains the standard protocol series (T1-weighted DCE sequence with 5 time points- before and 4 time points after contrast injection, and T2-weighted MRI with/without fat suppression) as well as the delayed contrast T1-images acquired 20-28 minutes after contrast administration and the calculated treatment response assessment maps (TRAMs). We recently introduced the utility of delayed-contrast MRI for calculating treatment response assessment maps (TRAMs) for improved specificity and accuracy in breast cancer diagnosis. The TRAMs are calculated by subtracting 3D T1-MRIs acquired 5 minutes post-contrast injection from those acquired ~22 minutes thus depicting the spatial distribution of contrast accumulation/clearance. Furthermore, the application of deep-learning methods to breast MRI has a potential to improve diagnosis as well as predict pathological features of the tumors and their response to therapy.
Data Access
Version 2: Updated 2024/04/15
SOP class UID was changed for all TRAM images from MR Image to Secondary Capture Image (623 series).
| Title | Data Type | Format | Access Points | Subjects | License | Metadata | |||
|---|---|---|---|---|---|---|---|---|---|
| Images, Segmentations | MR, SEG | DICOM | Download requires NBIA Data Retriever |
632 | 632 | 6,811 | 1,259,688 | CC BY 4.0 | View |
| Clinical data | Histopathology, Molecular Test, Classification, Demographic | XLSX | 200 | CC BY 4.0 | — |
Additional Resources for this Dataset
The NCI Cancer Research Data Commons (CRDC) provides access to additional data and a cloud-based data science infrastructure that connects data sets with analytics tools to allow users to share, integrate, analyze, and visualize cancer research data.
- Imaging Data Commons (IDC) (Imaging Data)
Citations & Data Usage Policy
Data Citation Required: Users must abide by the TCIA Data Usage Policy and Restrictions. Attribution must include the following citation, including the Digital Object Identifier:
Data Citation |
|
|
Daniels, D., Last, D., Cohen, K., Mardor, Y., & Sklair-Levy, M. (2024). Standard and Delayed Contrast-Enhanced MRI of Malignant and Benign Breast Lesions with Histological and Clinical Supporting Data (Advanced-MRI-Breast-Lesions) (Version 2) [dataset]. The Cancer Imaging Archive. https://doi.org/10.7937/C7X1-YN57 |
Acknowledgements
We would like to acknowledge the individuals and institutions that have provided data for this collection:
- This work was supported by the Israel Precision medicine partnership (IPMP) of the Israel Science Foundation (ISF), grant number 3400/19.
- Harmonization of the components of this dataset, including into standard DICOM representation, was supported in part by the NCI Imaging Data Commons consortium. NCI Imaging Data Commons consortium is supported by the contract number 19X037Q from Leidos Biomedical Research under Task Order HHSN26100071 from NCI.
Related Publications
Publications by the Dataset Authors
The authors recommended the following as the best source of additional information about this dataset:
No other publications were recommended by dataset authors.
Research Community Publications
TCIA maintains a list of publications that leveraged this dataset. If you have a manuscript you’d like to add please contact TCIA’s Helpdesk.